IN THIS LESSON
Learn some more details about chemical bonds.
1/4/25: I consider this lesson complete right now, but I intend to redraw the diagrams to be of higher quality.
Bonds form when orbitals overlap. There can be a maximum of two electrons (they must have opposite spins) in the overlapping orbitals. Maximum effective overlap is the maximum amount the orbitals can overlap without causing the atoms to repel from each other.
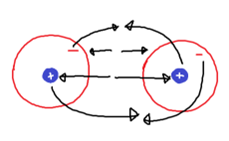
Atoms have both attractive and repulsive forces between them when they interact. The positively charged nuclei of the atoms repel each other and the negatively charged electron cloud of each one does the same. These are the repulsive forces. Additionally, the nucleus of one atom attracts to the electron cloud of the other, and vice versa. These are the attractive forces.
For example:
The sp³ hybridized orbital is a hybridization of one orbital from the s subshell and three orbitals from the p subshell in the valence shell. When the s and p shells merge into the sp³ shell, they form a shape similar to the tetrahedral molecular shape.
The sp² hybridized orbital is similar to the sp³ orbital, however it consists of one orbital from the s subshell and two orbitals from the p subshell in the valence shell. When these orbitals are hybridized, there will be one left normal from the p subshell.
Sigma (σ) vs Pi (π) Bonds
Sigma bonds are formed by the overlap of orbitals end-to-end. There is one in every bond. Pi bonds are formed by the overlap of orbitals side to side. There is one pi bond in every double bond and two pi bonds in every triple bond.
Bond Enthalpy
Breaking bonds requires, or takes in, energy. This process is endothermic and has a positive ΔH value. Atoms bonding together releases energy. This process is exothermic and has a negative ΔH value.
Bond Energy (aka Bond Dissociation Energy or D) is the enthalpy of when one mole of bonds is broken in gas phase. The D value is always positive.
The enthalpy of one of these reactions (ΔHᵣₓₙ) is equal to the sum of all the D values of bonds broken minus the sum of all the D values of bonds formed. To find these values, you need to know how to tell which bonds are broken in a reaction and which ones are formed.
For example:
C₆H₁₂O₆ + 6 O₂ → 6 CO₂ + 6 H₂O
Taking this chemical equation, we know that the bonds between the Os and Cs in the glucose are broken and the bonds between all the Cs are broken. The bonds formed are the ones between the Cs and the O₂s to form CO₂ and the Hs combining with OH groups to form H₂O molecules.