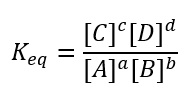IN THIS LESSON
Learn more about the equilibrium constant K.
1/4/25: I consider this lesson complete right now, but I am willing to edit it as needed.
The equilibrium constant (Keq) is a numerical representation of the relative amounts of products versus reactants in a system at equilibrium. Using a generic chemical equation, we can demonstrate this:
aA + bB → cC + dD
The lowercase letters represent the number of moles of the compounds, which are represented by the uppercase letters.
The equilibrium constant is calculated as:
The brackets represent the concentrations of the compound written inside them. The products are always written on the top of the equation and the reactants are always written on the bottom.
Keq or K is just the generic variable that represents the equilibrium constant. There are many more specific names, such as the following:
K꜀– specifically shows that the constant is calculated using concentrations
Kₚ – shows that the constant is calculated using partial pressures of gases
Kₐ – constant of a reaction in which an acid breaks
Kb – constant of a reaction in which a base breaks
Kₛₚ – constant of a reaction in which a solid dissolves in water
Kw – constant of the dissociation of water
Rules for writing and using K:
Products are always on top, reactants always on the bottom
Concentrations are expressed in M (mol/L, molarity)
Coefficients in the chemical equation are turned to exponents in the equation to find the constant
Solids and pure liquids are not included in the equation to find the constant
Kₚ = K꜀(RT)Δn (will be explained in later lessons) à sometimes Kₚ = K꜀, sometimes they are different
K for the reaction one way is equal to 1/K for the reaction in the other direction
Changes in concentration or pressure don’t affect K, only temperature does.
Information we can gather from a K value:
K > 1 – more products, considered “product-favored”
K < 1 – more reactants, considered “reactant-favored”
When K has reached around 10⁶ or higher, reaction goes virtually to completion
When K is about 10⁻⁶ or lower, reaction is essentially non-existent