IN THIS LESSON
Learn about a concept that is relevant to covalently bonded molecules.
1/4/25: I consider this lesson complete right now, but I intend to improve on the diagrams to improve quality.
Polarity occurs between two different covalently bonded atoms when there is a substantial difference in their electronegativities. In more complex molecules, they are polar when they are asymmetrical and the electronegativities of their atoms pull the electrons toward one side or one or a few atoms.
If the difference in electronegativity between two atoms is 0, the bond is non-polar. If it is between 0.1-1.9, the bond is polar. Anything higher is an ionic bond. Generally, an electronegativity difference of 0.4 or less is considered negligible; however, for our purposes, we will still count those differences as important.
To find the polarity of an individual bond, first find the difference in electronegativity between the two atoms. If it is within the polar range, proceed. Use a vector to show where the electrons are going to congregate; in other words, draw an arrow pointing from the less electronegative atom to the more electronegative atom. The atom with the arrow pointing toward it will have a partially negative charge, and the other will have a partially positive charge.
To find the polarity of a whole molecule, we use the polarity of individual bonds, the vectors representing these polarities, and the VSEPR molecular shapes.
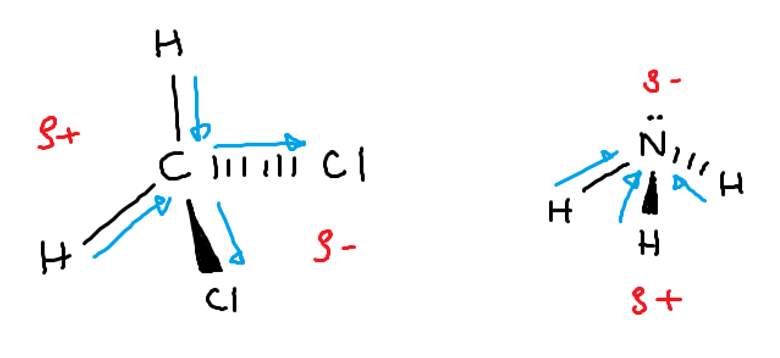
First, there are some basic rules to drawing molecules in 3D space. In most of our examples, molecules will have four electron pairs. Draw the central atom and two other atoms bonded to the central atom with simple lines, as before. To draw atoms that stick toward you, draw a colored-in thin triangle to connect the atom to the central one. To draw atoms that stick away from you, draw dashed lines between that atom and the central one.
Using these diagrams, we can begin by drawing the molecule of interest in the correct shape according to the VSEPR rules. Then, find the electronegativity of all the atoms involved. Use these values to see which ways the arrows indicating polarity point in each individual bond in the molecule. Each of these vectors represents a dipole. If all the dipoles are symmetrical (all pointing toward the center or all pointing away from the center), the molecule is non-polar.
For example:
If the dipoles point in different directions (are asymmetrical), the molecule is polar.
For example: