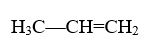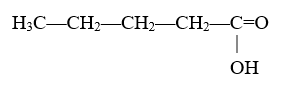IN THIS LESSON
Learn about the different elements of an organic molecule.
1/4/25: I consider this lesson complete right now, but I am willing to edit it as needed.
There are many functional group classes. These help determine what kind of functional group they are and how we should identify them in the names of organic molecules. The following classes are listed in order of importance. The most important functional groups present in a molecule are shown through the suffix of the molecule’s name. The functional groups after that are listed at the beginning as prefixes, decreasing in importance for each prefix.
Alkenes are functional groups with two carbon atoms bonded together with a double bond. They can be placed anywhere within a hydrocarbon molecule. Alkenes are always indicated with the “-ene” suffix. To name a molecule containing an alkene group: (1) number the carbons as indicated in the last lesson, (2) find the carbons that the double bond is in between and take the lower numbered one, (3) find the root word for the number of carbons, and (4) add the “-ene” suffix.
For example:
The longest carbon-to-carbon chain has three carbons, meaning we’ll use the prefix “prop-.” The alkene bond is placed between carbons 1 and 2, so we’d usually use the number 1. However, in this molecule, this is not necessary because changing where the double bond is would not change the molecule. So, we can disregard the placement-number in this case. Lastly, we add the suffix “-ene,” creating the final name: propene. Its alternate name with the number indicating the position is prop-1-ene.
Alkynes are similar to alkenes, except they are triple bonds. They are named the same way as alkenes, however they use the suffix “-yne” instead.
Carboxylic acids contain one carbon atom with one oxygen double-bonded to it and another oxygen-hydrogen group single bonded to the carbon. They are indicated by the suffix “-anoic acid” if there are no other more important functional groups present in a molecule. If there are, they use the “carboxy-” prefix.
For example:
There are five carbon atoms in the longest carbon chain, so this molecule will use the “pent-” prefix. Since there are no other more important functional groups, the molecule’s name will end in “-anoic acid.” The carboxylic acid group is technically attached at carbon 1, this could be called pent-1-anoic acid. However, since carboxylic acid groups can only be attached at the end of a chain, the position does not matter. This molecule will be called pentanoic acid.
The following groups will all continue to be named like the previous molecule. Specific differences will be noted.
Aldehydes contain one carbon atom with one oxygen double-bonded to it and a hydrogen atom single bonded to the carbon. They are indicated by the suffix “-al” when there are no other more important groups present and the prefix “oxo-” when there are.
Ketones contain one carbon atom with a double-bonded oxygen attached. This carbon will be placed between other carbons in the middle of a hydrocarbon chain. They are indicated by the suffix “-one” when no other more important groups are present and the “oxo-” prefix when there are. Since they can be placed anywhere on a molecule, in most cases, the position must be indicated in the name.
Alcohols or hydroxyls contain one oxygen atom bonded to a hydrogen atom. They are indicated by the suffix “-ol” when no other more important groups are present and the prefix “hydroxy-” when there are. They can also be placed anywhere on a molecule, so in most cases, the position of the group must be indicated in the name of the molecule.
Esters contain a carbon with a double-bonded oxygen attached and a single bonded oxygen attached to this carbon atom and another carbon atom. The second carbon atom may be attached to other atoms, such as hydrogens or more carbons. This part of the ester is an alkyl chain, meaning it gets named as such and used as a prefix for the molecule name. You take the number of carbon atoms in this branch and put the prefix for that number in front of the suffix “-yl.” For the rest of the name, exclude the carbon in the alkyl branch from the numbered carbons in the longest chain, proceed with naming the molecule using the number prefixes as usual. The suffix for esters is “-anoate.”
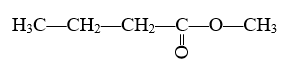
For example:
In this ester, the alkyl group is a carbon with three hydrogens attached. Since there is only one carbon here, this uses the prefix “meth-” and is called a “methyl” group. Excluding the carbon in the alkyl chain, there are four carbon atoms in the longest carbon chain in this molecule. This gives us the prefix “but-” which we add to the suffix “-anoate” and we get butanoate. Using “methyl” as a prefix like previously mentioned, this molecule’s final name is methyl butanoate.
Amines are a nitrogen molecule with two hydrogens attached. An amine group may be attached at any point on a hydrocarbon, meaning they may need a number to indicate their position on a hydrocarbon chain. They are indicated by the suffix “-amine” when there are no more important molecules present and the prefix “amino-” when there are.
Halogenohydrocarbons are singular halogen atoms attached to any carbon on a hydrocarbon chain. They may be attached at any point on a hydrocarbon chain so they may need a number to indicate their position on the molecule. They are indicated by the prefix that applies to the element they are or the “halo-” prefix.
Alkyls are a chain of carbons with hydrogens attached to fill the rest of the four necessary bonds of each carbon. They are named using the root word for their number of carbons in front of “-yl,” which is then applied as a prefix to the rest of the molecule name. The rest of the name is indicated by the number of carbons in the longest chain as usual, with the root word for this number and whichever suffix applies to the molecule.
For example:In this ester, the alkyl group is a carbon with three hydrogens attached. Since there is only one carbon here, this uses the prefix “meth-” and is called a “methyl” group. Excluding the carbon in the alkyl chain, there are four carbon atoms in the longest carbon chain in this molecule. This gives us the prefix “but-” which we add to the suffix “-anoate” and we get butanoate. Using “methyl” as a prefix like previously mentioned, this molecule’s final name is methyl butanoate.
Amines are a nitrogen molecule with two hydrogens attached. An amine group may be attached at any point on a hydrocarbon, meaning they may need a number to indicate their position on a hydrocarbon chain. They are indicated by the suffix “-amine” when there are no more important molecules present and the prefix “amino-” when there are.
Halogenohydrocarbons are singular halogen atoms attached to any carbon on a hydrocarbon chain. They may be attached at any point on a hydrocarbon chain so they may need a number to indicate their position on the molecule. They are indicated by the prefix that applies to the element they are or the “halo-” prefix.
Alkyls are a chain of carbons with hydrogens attached to fill the rest of the four necessary bonds of each carbon. They are named using the root word for their number of carbons in front of “-yl,” which is then applied as a prefix to the rest of the molecule name. The rest of the name is indicated by the number of carbons in the longest chain as usual, with the root word for this number and whichever suffix applies to the molecule.
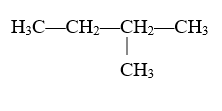
For example:
Since there is only one carbon in this alkyl group, it is called “methyl.” It is attached to carbon 2 of this hydrocarbon chain. The main carbon chain has four carbon molecules and is an alkane so it is called butane. The full molecule is called 2-methylbutane.
Note: numbers used to indicate positions of functional groups are placed in front of the functional group indicator (the relevant suffix or prefix) that they apply to.