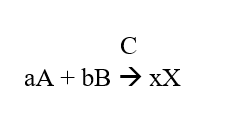IN THIS LESSON
Gain a basic understanding of chemical kinetics.
1/4/25: I consider this lesson complete right now, but I am willing to edit it as needed.
A rate of reaction is the measure of change (often in concentration) over time. Rates of reactions are always expressed as positive values. There are two important concepts to know about rates of reactions:
You must specify which product or reactant you are measuring.
The average rate of reaction is not the same as an instantaneous rate, which is the rate of the reaction at a specific point in time.
There are four major factors that affect reaction rates:
Concentration
Temperature
Catalysts
Particle size
These will be revisited soon.
Collision Theory is a theory that states that in order for two or more compounds to react, individual molecules must come in contact with each other, they must collide with enough energy to react, and must collide in the correct orientation to react.
A reaction coordinate is a graph of enthalpy of a reaction as it proceeds. They look like this:
The highest point on a reaction coordinate is the activated complex. The difference between the starting value of potential energy and the activated complex is the activation energy (Eₐ or Eₐ꜀ₜ), or energy that needs to be input into a reaction for the reaction to proceed. The difference between the starting value of potential energy and the ending value of potential energy is the ΔH value.
Now we will look at how each major factor affects rate, based on the new information we’ve just learned.
Higher concentration increases the chance of particle colliding. This increases reaction rate.
Higher temperature increases the kinetic energy of the particles. This causes the particles to collide with more energy, providing more activation energy for a reaction to occur. This increases reaction rate.
Catalysts decrease the amount of activation energy necessary for a reaction, making the number of collisions with enough energy to react increase. This increases reaction rate.
Smaller particle size provides more surface area for particles to react with one another and provides more opportunities for reactions. This increases reaction rate.
The Rate Equation helps us find the rate of reaction numerically. In the following equation:
a = number of moles of compound A
b = number of moles of compound B
x = number of moles of compound X
C is the catalyst present.
The rate equation itself is:
rate = k[A]ᵐ[B]ⁿ[C]ᵖ…
Where k is a rate constant at a given temperate and m, n, and p are experimentally determined values related to reaction order. However, for this class, we’ll stay focused on simple first order reactions. The equation for those looks like this:
rate = k[N]¹…
As N changes, so does the rate.