IN THIS LESSON
Learn about some unique organic molecule structures.
1/4/25: I consider this lesson complete right now, but I am willing to edit it as needed.
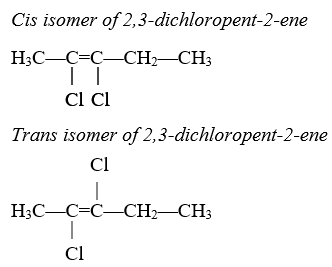
The main effects that double bonds have on alkenes are: (1) they shorten the distance between the carbon atoms they are bonding, (2) they increase the reactivity of the bond, and (3) they inhibit the rotation of attached functional groups, effectively locking them in a certain formation. This locking of the functional groups allows for cis/trans isomerism, where the molecules are almost the same in structure, but functional groups may be extending away from the main hydrocarbon chain in different directions.
Cis isomers have the functional groups extending in the same direction away from the hydrocarbon chain both in front of and after the double bond. Trans isomers have the functional groups extending in opposite directions away from the hydrocarbon chain in front of and after the double bond.
For example:
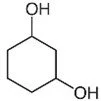
To name a molecule in a ring structure that has functional groups attached, number the carbons starting from the most important functional group, then work your way around the ring. Keep in mind that the functional groups should all have the lowest number possible. Then, use the numbers to indicate the positions of the functional groups by placing them in front of the applicable prefixes for the functional groups. Remember that these prefixes should go in front of “cyclo____ane.”
For example:
To name this molecule, you’d begin numbering the carbons from the top-most one, then work your way around clockwise. This would make the numbers of the carbons where the two alcohol groups are attached carbons 1 and 3. There are six carbon atoms in this ring structure, so the end of the name would be cyclohexane. Since there are two alcohol groups, the “dihydroxy-” prefix would apply to those groups. This gives us the name 1,3-dihydroxycyclohexane.
The special case with ring structures is benzene ring structures. They are named similarly to the cycloalkanes, however the word “cycloalkane” in the molecule name is replaced with “benzene.”